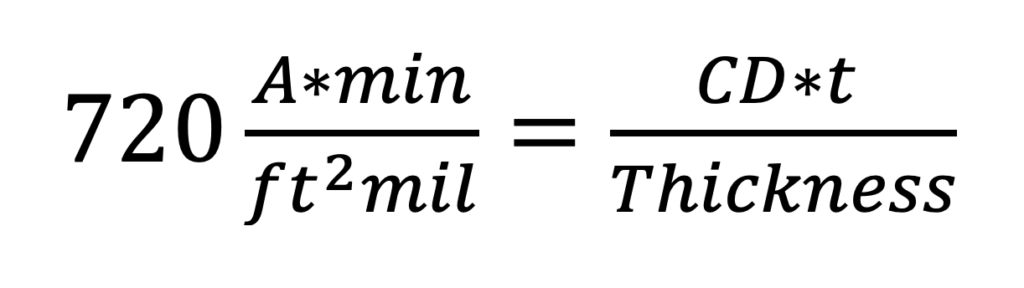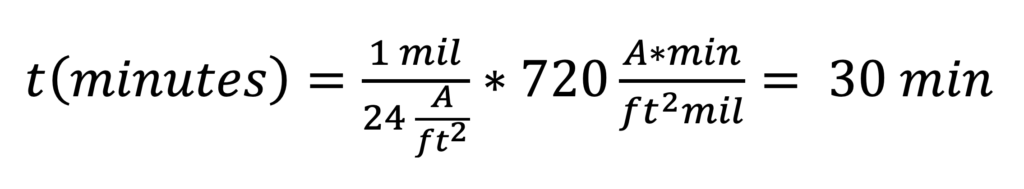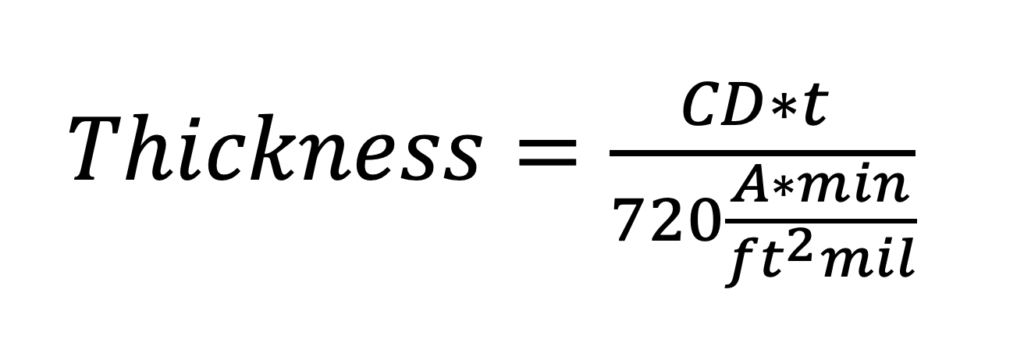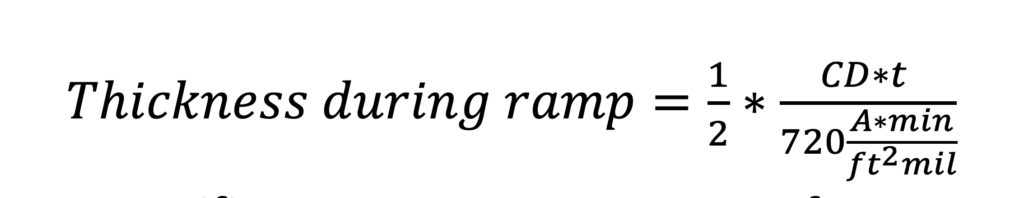The 720 Rule and Its Application to Current Density Anodizing
By Dr. Sjon Westre,
Introduction
CHEMEON brings anodizers and manufacturers in from around the country to teach them the best practices for aluminum anodizing. During the class, the 720 Rule is typically discussed along with current density anodizing. The 720 Rule is a very powerful tool for managers, estimators, and planners as well as anodizers but we often find that our students are not entirely familiar with what the 720 rule actually is, or what they can do with it.
What is the 720 Rule?
The 720 rule describes the relationship between the amount of current passed through an aluminum surface and the resultant anodic oxide thickness produced that is over time.
It is known that 720 Amp-minutes of current per square foot of load are required to produce one mil (0.001” or 25.4 micron) of anodic oxide (Westre 1997, 2017) (Westre 2000). Although the source of this relationship is not well documented, it has been suggested that the relationship is derived from the Ilkovic equation using the half-cell potential for aluminum. (Runge-Marchese 1999).
Use of the 720 Rule to predict anodizing behavior
At its most basic, the 720 Rule is a way to determine anodizing build rate, anodizing time, or the current density of the anodizing load when the other two parameters are known.
Functionally we express the relationship as:
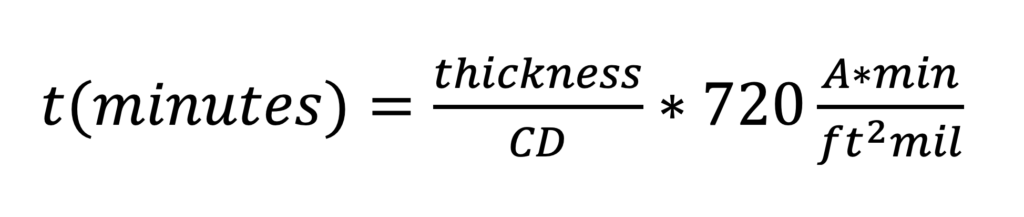
Where CD is the current density in amps per square foot, t is the exposure time in minutes, and the resulting thickness is in mils. We can rewrite Equation 1 to emphasize the relationships of interest:
Equation 2 allows us to calculate that to achieve a 1 mil oxide thickness at 24 amps per square foot (ASF), 30 minutes is required.
Table 1 shows the calculated time to 1 mil thickness for some common current densities.
Table 1. Anodizing times as a function of current density
| Current Density/amps per square foot | Time to 1 mil/min |
| 12 | 60 |
| 18 | 40 |
| 24 | 30 |
| 30 | 24 |
| 40 | 18 |
Simple rearrangement of Equation 2 shows that the amount of oxide generated at a particular current density as a function of time is:
For anodizing in sulfuric acid electrolytes, this provides a very good estimate regardless of alloy, electrolyte concentration, and/or electrolyte temperature. In other words, it can be used to estimate the behavior of Type II anodizing on 6061, as well as Type III anodizing on 2024. It has been noted that perhaps the 720 rule does not do as good of a job estimating 2024 thicknesses (Chesterfield 2008)
Compensating for ramp time when using the 720 Rule
For precision work, we must compensate for the amount of oxide added during the ramp period.
Equation 2 provides the anodizing rate at a constant current. In real world situations, the rectifier is ramped from zero current to the target final current density. What happens during the initial ramp of the rectifier? It turns out this is quite simple for linear ramps. If the current is increasing over time, t, to a final current density, CD, the amount of oxide generated during the ramp is
For example, if we ramp our rectifier to 30 amps per square foot over a 5 minute period, the ramp contributes 0.104 mil to the total thickness.
Equation 4 can be derived graphically by realizing that during a ramp, the area under the line in a current density vs time plot is a triangle whose area is 0.5 t*CD. Or, that the average current density during ramp, is one half the target final current density.
Another way to think about ramp compensation is that the amount of thickness formed during the ramp is half that would be formed during the same amount of time under full current density. This allows us to simplify our thinking when trying to predict total process time to produce a desired coating thickness.
Applications of the 720 rule
Estimating process times
Most of the time, we are interested in how long it will take to form a coating of a certain thickness at a given current density. Equation 2 allows us to estimate the amount of time to produce a given oxide thickness at a particular current density. We can compensate for the amount of oxide formed during the ramp by looking at Equation 4, and realizing that during the ramp, the average current density is half the final current density.
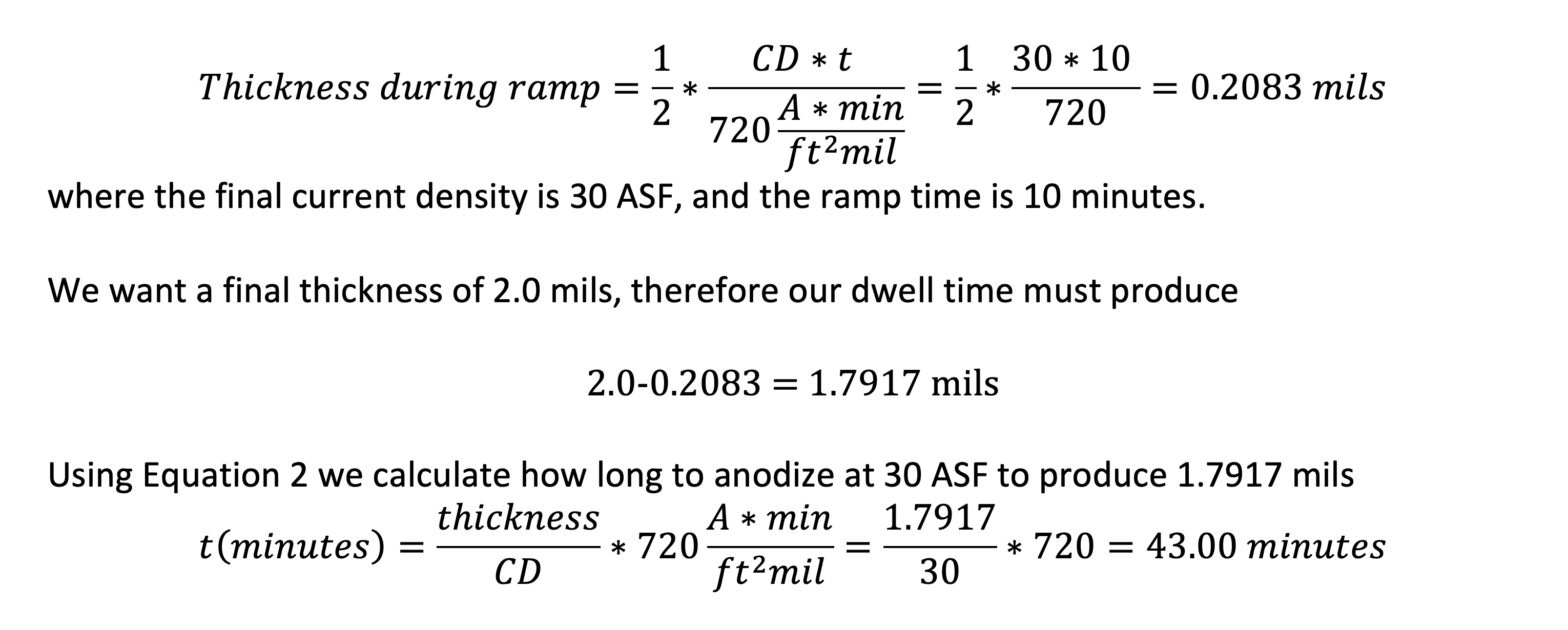
Using Equation 2 or Table 1, we find that if we want to put on 1 mil of oxide at 30 amps per square foot, it will take 24 minutes. So then we know that if we are doing MIL-SPEC anodizing to MIL-A-8625 and want to put on 2.0 mils, it will take 48 minutes anodizing at 30 ASF. What if we have a 10 minute ramp to the final current density? We can work successively through Equation 4 to deal with the ramp and then use Equation 2 to predict how long we have to run at full current density to achieve our target thickness of 2.0 mils.
From Equation 4
where the final current density is 30 ASF, and the ramp time is 10 minutes.
We want a final thickness of 2.0 mils, therefore our dwell time must produce
2.0-0.2083 = 1.7917 mils
Using Equation 2 we calculate how long to anodize at 30 ASF to produce 1.7917 mils
To summarize this example, a ramp of 10 minutes to 30 ASF with a dwell time of 43 minutes at 30 ASF will produce 2.0 mils of oxide to a good approximation.
The easy way
If one is comfortable with Equation 2, and the fact that during the ramp the effective current density is half that during the dwell period (Equation 4), there is a simpler way to predict the amount of time required to ramp and dwell.
- Use Equation 2 to predict the dwell time required to produce the coating thickness desired at the proposed current density.
- Subtract half the ramp time from the prediction in Equation 2 and use that as the ramp compensated dwell time.
Mathematically, a 10 minute ramp from zero to full dwell current produces the same effect as a 5 minute dwell at full current.
Example – Type II anodize run worked problem
Say an anodizer wishes to run Type II and then dye the parts deep black color. She might decide to run at 12 amps per square foot to a thickness of 1 mil. For various reasons – part configuration, racking density, etc. the anodizer chooses a 6 minute ramp. How long does the anodize need to dwell so that the final thickness is 1.0 mil?
Equation 2 or Table 1 shows us that at 12 ASF, 60 minutes are required. Our 6 minute ramping from zero to full current is equivalent to 3 minutes at full current density. Therefore 60-3=57 minutes of dwell at 12 ASF are required after the 6 minute ramp in order to achieve a 1.0 mil oxide thickness.
Bibliography
Westre, S.G. 1997, 2017. Anodizing Best Practices and Troubleshooting. Training Manual, Minden: CHEMEON Surface Technology.
Westre, S.G. 2000. Anodizer’s Reference Manual. Training Manual, Minden: CHEMEON Surface Technology.
Runge-Marchese, J.M. 1999. Electrochemical deposition of a composite polymer metal oxide. United States of America Patent 5,980,723. November 9.
Chesterfield, L. 2008. Calculating Andizing Rate for Type II and Type III Coatings. August 1. Accessed July 5, 2017. http://www.pfonline.com/articles/calculating-anodizing-rate-for-type-ii-and-type-iii-coatings.